Find the best dentist near you
Dentists play an important role in keeping your smile healthy. To care for teeth one must choose the dentist which can solve your teeth problems.
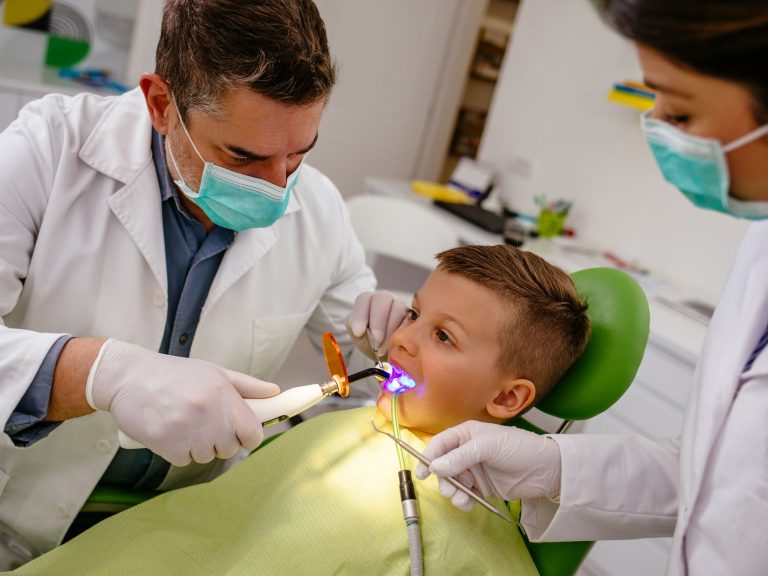
Dental Implant
We know about your hectic schedule. We also know the only way you truly understand a subject is by practicing it in a real environment. This is why we’ve set a playground area that’s full of hours of exercises, questions and challenges. It even has a gaming section.
Teeth Whitening
Whiter teeth and brighter smile not just helps you to build confidence but also help you in keeping a good healthy dental hygiene. Clinical teeth whitening treatment can last up to 5 years.
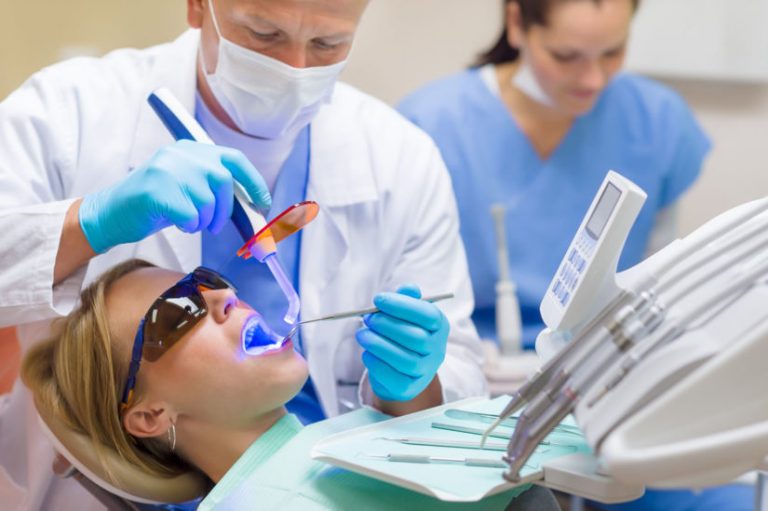





Our professional team
Our team Labmatters Digital works around the clock to gather information from the world and bring it to you. We have professionals who verify all the details before publishing for our viewers.
